Is Matter Around Us Pure?
Pure Substance
Pure substances cannot be broken into simpler forms by physical processes.
Elements
An element is a pure substance from which it cannot be purified by any physical or chemical method.
eg: - Oxygen (O), Hydrogen (H), Nitrogen (N), etc.
- Metals: Iron, Mercury, Copper, Gold, Silver, Platinum, Sodium, etc.
- Non-metals: Carbon, Oxygen, Sulfur, Nitrogen, Hydrogen, etc.
- Metalloids: Boron, Silicon, Germanium, etc.
Compounds
The substance formed after mixing two or more elements in a definite proportion in a chemical combination is called a compound.
eg:- Water (H2O), Carbon dioxide (CO2), Ammonia (NH3), etc.
Impure Substances
Impure substances can be broken down into simpler substances by physical processes.
Mixture and its Types
It is a substance in which two or more substances are simply mixed together in any proportion.
eg:- Air is a mixture of Oxygen(O), Nitrogen(N), Co2, and water vapour
Mixtures are of two types:
- Homogenous Mixture:A mixture in which the compostion in uniform is called hemogeneous mixture. Example: Sugar in water H has uniform composition.
- Heterogenous Mixture: A mixture in which the compostion is not uniform throught the mixture. Example: Water and sand, Air.
Properties of Mixtures
| Property | Solution | Colloid | Suspension |
|---|---|---|---|
| Particle Size | Less than 1 nm | 1 to 1000 nm | More than 1000 nm |
| Appearance | Clear | Cloudy | Cloudy |
| Separation | Does not separate | Does not separate | Separates or settles |
| Filterability | Passes through filter paper | Passes through filter paper | Particles do not pass through filter paper |
| Effect of Light | Light can pass through | Scatters light | Light cannot pass through |
| Example | Salt solution | Mayonnaise | Muddy water |
Tyndall Effect
The Tyndall effect is light scattering by particles in a colloid or in a very fine suspension. It is seen only in colloids and some suspensions.
Components of Different Types of Mixtures
- Solution: Solute + Solvent
- Colloid: Dispersed phase + Medium
- Suspension: Suspended particles
Solution Components
- Solvent: The component that dissolves the other component (usually present in larger amount).
- Solute: The component that is dissolved in the solvent (usually present in lesser quantity).
Concentration of a Solution
- Saturated Solution: No more solute can be dissolved at a given temperature.
- Unsaturated Solution: More solute can be dissolved at a given temperature.
- Solubility: The amount of solute present in a saturated solution at a given temperature.
The concentration of a solution is the amount of solute present in a given amount (mass or volume) of solution.
Concentration of Solution:
Amount of solute / Amount of solvent or
Amount of solute / Amount of solution
Two methods to find the concentration of a solution:
- Mass by mass percentage of a solution = (Mass of solute / Mass of solution) × 100%
- Mass by volume percentage of a solution = (Mass of solute / Volume of solution) × 100%
Types of Colloids
Trick to remember: Gas is two times, Liquid is three times, and Solid is three times. Now to remember type: Subodh Sharma Geti) Fir Se England fir America Aaye
| Dispersing Medium | Dispersed Phase | Type | Example |
|---|---|---|---|
| Gas | Solid | Aerosol | Smoke |
| Gas | Liquid | Aerosol | Fog |
| Liquid | Solid | Sol | Paint |
| Liquid | Liquid | Emulsion | Milk |
| Liquid | Gas | Foam | Whipped Cream |
| Solid | Solid | Solid Sol | Alloy |
| Solid | Liquid | Gel | Jelly |
| Solid | Gas | Foam | Pumice Stone |
Separation Methods
In both real life and chemistry, people (or components) separate only when there are differences between them such as thinking, emotions, compatibility, etc. In mixtures, we separate the components based on their physical property differences using specific techniques.
Different Types of Separation Methods
- Filtration (Difference in particle size)
- Magnetic Separation
- Sublimation
- Separating Funnel
- Evaporation
- Crystallization
- Distillation
- Fractional Distillation
- Chromatography
- Centrifugation
1. Filtration
Principle: Based on the difference in particle size.
Application: Used to separate solids from liquids or gases.
Example: Filtering coffee grounds from brewed coffee.
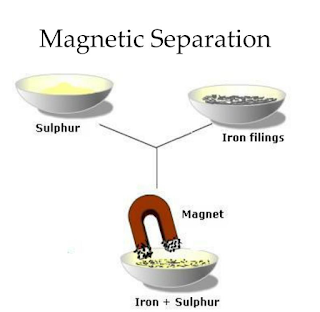
2. Magnetic Separation
Principle: Utilizes magnetic properties to separate magnetic materials from non-magnetic ones.
Application: Separating iron filings from sand.
3. Sublimation
Principle: Separates a substance that sublimes (changes directly from solid to gas) from a mixture.
Example: Separating iodine from a mixture containing sand.
4. Separating Funnel
Principle: Used to separate immiscible liquids (liquids that do not mix).
Example: Separating oil and water.
5. Evaporation
Principle: Used to separate a soluble solid from a liquid by evaporating the liquid.
Example: Obtaining salt from seawater.
6. Crystallization
Principle: Used to obtain pure solid particles from a solution by allowing crystals to form.
Example: Purification of salt from impure salt solutions.
7. Distillation
Principle: Separates mixtures of liquids based on their boiling points.
Example: Separating alcohol from water.
8. Fractional Distillation
Principle: Similar to distillation but used for separating components with closer boiling points.
Example: Separating different fractions of crude oil.
9. Chromatography
Principle: Separates different components in a mixture based on their movement through a stationary phase.
Application: Analyzing colors in dyes, separating pigments, etc.
10. Centrifugation
Principle: Uses centrifugal force to separate suspended particles from a liquid.
Application: Separating cream from milk.






0 Comments